


| Media Release | 13 June 2017 |
Pharmaceutical research company Pharmaxis (ASX: PXS) today announced its recently completed international Phase 3 trial of Bronchitol® (mannitol) in adults with cystic fibrosis (CF) met its primary endpoint.
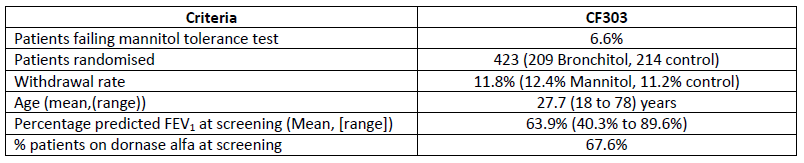
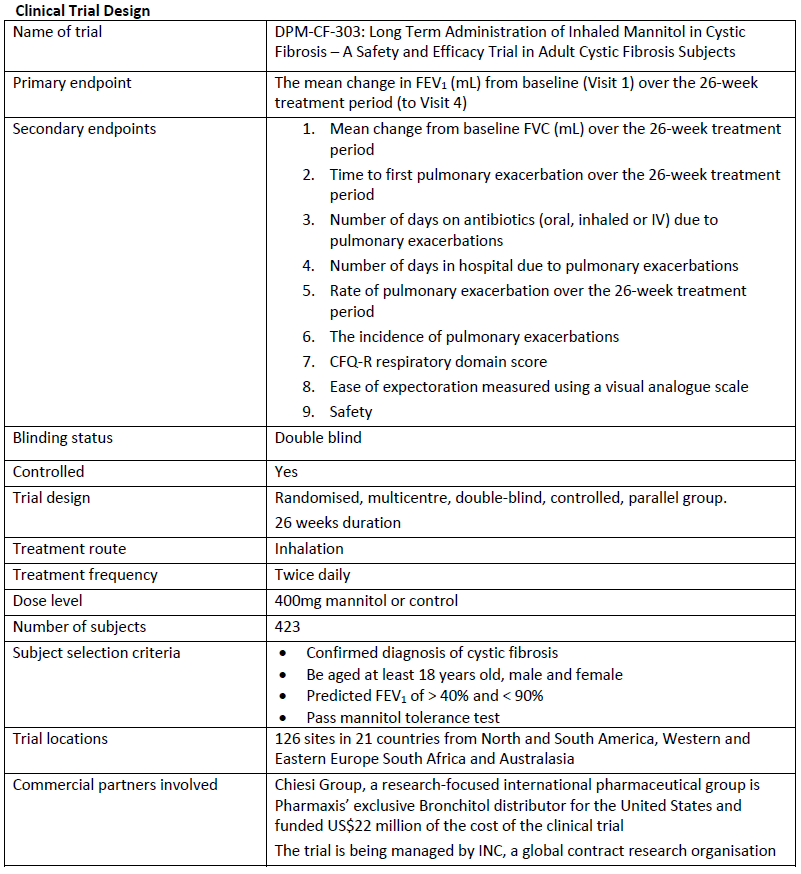
The clinical trial (CF303) was designed after extensive consultation with the US Food and Drug Administration (FDA) in order to gain marketing approval for Bronchitol to treat adult CF patients in the United States. The trial was a 26-week randomized, double-blind parallel group investigation of Bronchitol administered twice daily in CF patients aged 18 and over to assess improvements in lung function and other parameters, as well as safety. The trial recruited a total of 423 patients across 126 sites in 21 countries including North and South America, Western and Eastern Europe and Australasia. More than a quarter of patients were from the USA.
Pharmaxis Chief Executive Officer Mr Gary Phillips said, "I am pleased that the study met its primary endpoint and whilst the effect size is reduced relative to previous studies Pharmaxis and its US partner Chiesi believe the results are sufficient to underpin a resubmission of the Bronchitol New Drug Application to the FDA which we expect will occur in 2018. Incremental improvements in the standard of care for CF have resulted in longer life expectancy and adult patients now exceed 50% of the CF population in many countries. Adult CF patients who experience deteriorating health or difficulty in complying with existing medications continue to require access to new treatment options and in this trial Bronchitol brought benefit to patients on top of their existing treatment regimen and had a good safety profile."
"The study results reinforce the body of clinical trial evidence for Bronchitol and I'd like to thank both the global CF community and the Pharmaxis team who have delivered a very high quality study with low rates of patient withdrawal in both treatment arms."
Pharmaxis has partnered its work on Bronchitol for the United States with Chiesi Group (Chiesi), a global pharmaceutical company headquartered in Parma, Italy. Chiesi USA, the American affiliate of Chiesi Group is responsible for completing and filing the updated Bronchitol NDA with the FDA.
Mr Ken McBean, President and Chief Executive Officer of Chiesi USA said, "The conclusion of this key pivotal trial, conducted as a partnership between Pharmaxis and Chiesi R&D teams, provides a foundation for moving towards FDA approval, recognizing the challenge of the agency scrutiny of such assets. We hope, in turn, it can also leverage this new therapeutic option to the adult CF population in the US. If successful, it will also provide a further step towards the growth of our existing portfolio and our business vision in the valuable US market, where our presence is focused on specialty care opportunities including in the field of respiratory medicine, and where we continue to grow through internal pipeline execution and judicious partnering with biotech and pharma companies."
Mr Phillips added, "Our aim in assigning Bronchitol to distributers in existing markets, launching in new markets like Russia and partnering this study to gain access to the US market has been to manage our spend whilst increasing overall volumes. We believe the Bronchitol business segment based out of our manufacturing facility in Sydney will transition to profitability over the next 12 to 24 months irrespective of any approval in the US. The cash that this will return will help fund Pharmaxis' drug discovery activities and the exciting pipeline of assets we have developed."
Additional data from the trial will be presented at the North American Cystic Fibrosis Conference in Indianapolis, Indiana on 2 - 4 November, 2017.
Results
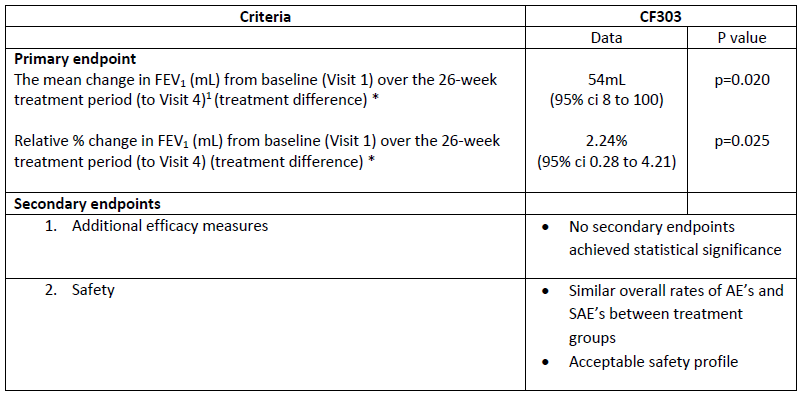
* The result on the primary endpoint has been confirmed in several sensitivity analyses
Demographics
SOURCE: Pharmaxis Ltd, Sydney, Australia
CONTACT: Felicity Moffatt, phone +61 418 677 701 or email felicity.moffatt@pharmaxis.com.au
About Pharmaxis
Pharmaxis (ACN 082 811 630) is an Australian research pharmaceutical company focused on inflammation and fibrosis with a portfolio of products at various stages of development and approval. Its product Bronchitol® for cystic fibrosis is marketed in Europe, Russia and Australia. Its product Aridol® for the assessment of asthma is sold in Europe, Australia and Asia. The company's development pipeline is centred on its expertise in amine oxidase chemistry and includes a series of Lysyl Oxidase Inhibitors that will enter clinical development in 2017 targeting fibrotic diseases of the heart, kidney, liver and lung. In May 2015, Boehringer Ingelheim acquired the Pharmaxis investigational drug PXS-4728A, a potent inhibitor of Semicarbazide-Sensitive Amine Oxidase (SSAO), to develop it for the treatment of the liver-related condition Non-alcoholic Steatohepatitis (NASH) and other inflammatory diseases. Pharmaxis is listed on the Australian Securities Exchange (symbol PXS). The company's head office, manufacturing and research facilities are located in Sydney, Australia. For more information about Pharmaxis, please see www.pharmaxis.com.au
About Chiesi Group
Based in Parma, Italy, Chiesi is an international research-focused Healthcare Group, with over 80 years of experience in the pharmaceutical industry, present in 26 countries. Chiesi researches, develops and markets innovative drugs in the respiratory therapeutics, specialist medicine and rare disease areas. In 2016, Chiesi achieved sales of over 1.5 billion Euros, constituting 7% growth over 2015. Its R&D organization is headquartered in Parma (Italy), and integrated with 6 other key R&D groups in France, the USA, the UK, Sweden and Denmark to advance Chiesi's pre-clinical, clinical and registration programmes. Chiesi employs nearly 5,000 people.
For more information please visit www.chiesi.com
About Chiesi USA
Chiesi USA, Inc., headquartered in Cary, N.C., is a specialty pharmaceutical company focused on commercializing products for the hospital and adjacent specialty markets. Key elements of the Company's strategy are to focus its commercial and development efforts in the hospital and adjacent specialty product sector within the U.S. pharmaceutical marketplace; continue to seek opportunities to acquire companies, marketed or registration-stage products and late-stage development products that fit within the Company's focus areas; and generate revenues by marketing approved generic products through the Company's wholly-owned subsidiary, Aristos Pharmaceuticals, Inc. Chiesi USA, Inc. is a wholly-owned subsidiary of Chiesi Group. For more information, visit www.chiesiusa.com.
About Bronchitol
Bronchitol is a precision spray-dried form of mannitol, delivered to the lungs by a specially designed, portable inhaler. Bronchitol works by rehydrating the airway/lung surface and promoting a productive cough. The product is approved for marketing for the treatment of cystic fibrosis patients aged over six years in Australia and Russia and for patients aged 18 years and over throughout the European Union and in Israel.
Forward-Looking Statements
Forward-looking statements in this media release include statements regarding our expectations, beliefs, hopes, goals, intentions, initiatives or strategies, including statements regarding the potential of products and drug candidates. All forward-looking statements included in this media release are based upon information available to us as of the date hereof. Actual results, performance or achievements could be significantly different from those expressed in, or implied by, these forward-looking statements. These forward-looking statements are not guarantees or predictions of future results, levels of performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results to differ materially from those expressed in the statements contained in this document. Except as required by law we undertake no obligation to update these forward-looking statements as a result of new informbation, future events or otherwise.
| © Copyright 1996-2019 irasia.com Ltd. All rights reserved. |
|
DISCLAIMER: irasia.com Ltd makes no guarantee as to the accuracy or completeness of any
information provided on this website. Under no circumstances shall irasia.com Ltd be liable
for damages resulting from the use of the information provided on this website.
TRADEMARK & COPYRIGHT: All intellectual property rights subsisting in the contents of this website belong to irasia.com Ltd or have been lawfully licensed to irasia.com Ltd for use on this website. All rights under applicable laws are hereby reserved. Reproduction of this website in whole or in part without the express written permission of irasia.com Ltd is strictly prohibited. TERMS OF USE: Please read the Terms of Use governing the use of our website. |