
Pharmaxis Ltd


| Media Release | 21 May 2013 |
Pharmaceutical company Pharmaxis (ASX: PXS) today announced it had concluded a productive end of review meeting with the US Food and Drug Administration (FDA) which has provided the Company with a clear outline of the clinical trial required to gain approval for Bronchitol® (mannitol) to treat cystic fibrosis (CF) in the United States.
The Type A meeting discussed the remaining clinical work required to show substantial evidence of efficacy in patients with cystic fibrosis. The FDA and Pharmaxis agreed that the clearest and most expeditious regulatory path forward is to conduct a further single pivotal trial in adults aged 18 years and over. It was also agreed that the trial should have a very similar design to the two large scale clinical trials already undertaken by Pharmaxis (CF 301 and CF 302). It will be of six months duration with improvement in lung function as measured by a change in Forced Expiratory Volume in 1 second (FEV1) as its primary endpoint.
Pharmaxis CEO Mr Gary Phillips said, "The results of the meeting with the FDA are very pleasing. With the guidance of the FDA we have been able to quickly remove uncertainty about what is required to gain approval for Bronchitol in the US market where orphan drug status allows us seven years of market exclusivity from approval.
| "Our aim was to identify the fastest way to the US market with the least clinical and regulatory risk. Restricting the trial population to adult cystic fibrosis patients has a number of benefits compared to the wider population studied in the earlier phase 3 trials. The variability of FEV1 results and the apparent response to the control drug seen in children and adolescents will no longer be an issue. A post hoc analysis1 of the subgroups of adult patients in CF301 and CF302 showed a significant improvement in FEV1 and there was no evidence of a control effect. We will therefore use respirable low dose mannitol as the control." | 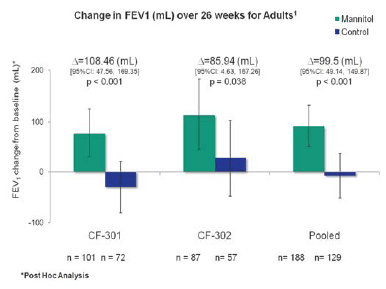 |
Pharmaxis will utilise its extensive clinical database from CF301 and CF302 to ensure the relevant adult patient group is enrolled, design effective patient retention strategies and accurately power the trial. At this stage Pharmaxis anticipates that the number of subjects will be around 300, which is similar to the previous clinical trials. Pharmaxis will propose a separate development program to the FDA for patients aged six to 17 years after the protocol for the study in adult patients is finalised.
Mr Phillips added, "The outcome of the meeting means a lot of the uncertainty about the cost and time involved to get Bronchitol approved for use in the USA for CF has been removed and its value as an asset to Pharmaxis confirmed. We can now confidently complete the Company's business plan based on firm foundations and I look forward to providing detail of the plan on 28th May."
The Type A meeting followed a Complete Response Letter issued by the FDA in March 2013 advising Pharmaxis that another clinical trial would be required in order to secure approval for Bronchitol. Bronchitol is approved in Europe for adults and in Australia for patients aged six years and over and is reimbursed for patients in Germany, Australia, Denmark and the United Kingdom.
1. Ref: Bilton D, Robinson P, Cooper P, et al. J Cystic Fibrosis 2011, Suppl 20 (A78) and Data on File Pharmaxis
SOURCE: Pharmaxis Ltd, Sydney, Australia
CONTACT: Felicity Moffatt, phone +61 418 677 701 or email felicity.moffatt@pharmaxis.com.au
About Pharmaxis
Pharmaxis (ACN 082 811 630) is a specialist pharmaceutical company involved in the research, development and commercialization of therapeutic products for chronic respiratory disorders. Its product Aridol® for the assessment of asthma is sold in key international markets. Its product Bronchitol® for cystic fibrosis is recently launched in Europe and Australia and its development pipeline of products includes, Bronchitol for bronchiectasis, PXS64 for the treatment of lung fibrosis, ASM8 for asthma and PXS4728 for fibrotic disease. Pharmaxis is listed on the Australian Securities Exchange (symbol PXS). The company's head office and manufacturing facilities are located in Sydney. For more information about Pharmaxis, go to www.pharmaxis.com.au or contact Investor Relations on phone +61 2 9454 7200.
About Bronchitol
Bronchitol has been developed to help clear mucus (a major source of lung infections), improve lung function and reduce exacerbations in patients with cystic fibrosis. Bronchitol is a proprietary formulation of mannitol administered as a dry powder in a convenient hand-held inhaler. Inhaled mannitol hydrates the lungs, helps restore normal lung clearance, and allows patients to clear mucus more effectively.
About Cystic Fibrosis
In a healthy person, there is a constant flow of mucus over the surfaces of the air passages in the lungs, removing debris and bacteria. In CF, an inherited disease, a defective gene disrupts ion transport across the epithelial membrane within cells. In the lungs, this leads to a depletion of the airway surface liquid that normally bathes the cilia, and a resultant reduction in mucociliary clearance. The result is thick, sticky mucus that clogs the lungs, severely restricting the natural airway-clearing process. It also increases the potential for bacteria to become trapped and for inflammation, thus creating an unhealthy lung environment that leads to life-threatening lung infections.
Forward-Looking Statements
Forward-looking statements in this media release include statements regarding our expectations, beliefs, hopes, goals, intentions, initiatives or strategies, including statements regarding the potential for Bronchitol. All forwardlooking statements included in this media release are based upon information available to us as of the date hereof, and we assume no obligation to update any such forward-looking statement as a result of new information, future events or otherwise. We cannot guarantee that any product candidate will receive regulatory approval or that we will seek any such approval.
| © Copyright 1996-2023 irasia.com Ltd. All rights reserved. |
|
DISCLAIMER: irasia.com Ltd makes no guarantee as to the accuracy or completeness of any
information provided on this website. Under no circumstances shall irasia.com Ltd be liable
for damages resulting from the use of the information provided on this website.
TRADEMARK & COPYRIGHT: All intellectual property rights subsisting in the contents of this website belong to irasia.com Ltd or have been lawfully licensed to irasia.com Ltd for use on this website. All rights under applicable laws are hereby reserved. Reproduction of this website in whole or in part without the express written permission of irasia.com Ltd is strictly prohibited. TERMS OF USE: Please read the Terms of Use governing the use of our website. |