
Pharmaxis Ltd


| Media Release | 13 December 2011 |
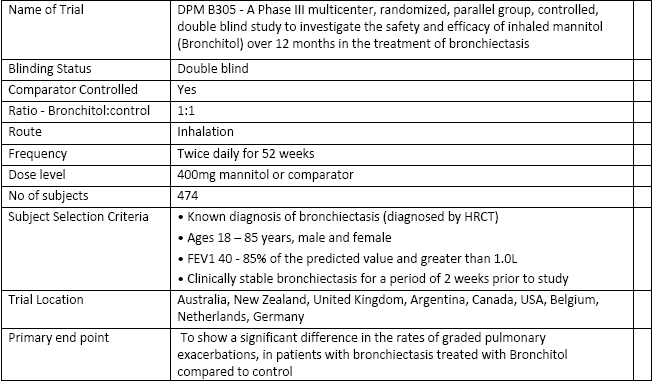
Pharmaceutical company Pharmaxis (ASX: PXS) is pleased to announce that its Phase III, DPM-B-305 study of inhaled Bronchitol, for the treatment of people with bronchiectasis, has reached its pre-specified recruitment target.
This major Phase III clinical trial received input from both the U.S. and the EU regulators on its design. The trial will show if treatment with Bronchitol over twelve months leads to a reduction in pulmonary infectious episodes for people with bronchiectasis. In addition, the trial will collect data on quality of life, lung function and other aspects of the condition.
The trial is being conducted at 83 hospitals throughout the world and participants receive either active drug (Bronchitol) or a comparator for a total of twelve months.
"Reaching the enrollment target has involved a great deal of effort by many people and we are delighted to have come to this point in the trial" said Pharmaxis Chief Executive Officer Dr Alan Robertson. "The extended bronchiectasis community has been extremely supportive throughout the trial and we are all looking forward to having the drug available."
There have been no new therapeutic advances for this patient group in the last twenty years. Bronchiectasis is an incurable, degenerative and chronic lung condition affecting more than 200,000 people in Europe alone. Bronchitol is expected to be the first targeted medication for this population and addresses a fundamental medical need.
In the United States, at least 110,000 people are receiving treatment for bronchiectasis, medical-care expenditure is over US$630 million per year and patients themselves spend between US$6,000 and US$13,000 on treatment. Widespread availability of high resolution scanners is leading to increases in diagnosis rates and bronchiectasis is more common than previously thought. Pharmaxis is developing Bronchitol as a daily treatment administered by inhalation to the patient's lungs.
The trial results will be available during the early part of 2013.
| SOURCE: | Pharmaxis Ltd, Sydney, Australia |
| CONTACT: | Alan Robertson - Chief Executive Officer Ph: +61 2 9454 7200 or email alan.robertson@pharmaxis.com.au |
RELEASED THROUGH:
Australia:
Felicity Moffatt, phone +61 418 677 701 or email felicity.moffatt@pharmaxis.com.au
About the trial
The following information is provided in accord with the ASX and AusBiotech Code of Best Practice for Reporting by Life Science Companies.
About Bronchitol
Pharmaxis Ltd is developing Bronchitol for the management of chronic obstructive lung diseases including bronchiectasis and cystic fibrosis. Bronchitol is a proprietary formulation of mannitol administered as a dry powder in a convenient hand-held inhaler. It is designed to hydrate the lungs, restore normal lung clearance mechanisms, and help patients clear mucus more effectively. Clinical studies have shown Bronchitol to be well tolerated, to improve quality of life, and to stimulate mucus hydration and clearance in people with bronchiectasis and cystic fibrosis.
About Bronchiectasis
Bronchiectasis is one of the chronic obstructive pulmonary diseases, or COPDs, and affects children and adults. It is often mistaken for asthma or pneumonia and misdiagnosis is common. In this disease the bronchial tubes become irreversibly enlarged, forming pockets that can become infected. The bronchi walls are damaged, causing impairment to the lung's complex cleaning system. The tiny hairs, or cilia, which line the bronchial tubes and sweep them free of dust, germs and excessive mucus are unable to function properly. The result is that matter such as mucus and bacteria accumulates affecting the performance of the lungs and the quality of life of the individual.
About Pharmaxis
Pharmaxis (ABN 75 082 811 630) is a specialist pharmaceutical company involved in the research, development and commercialization of therapeutic products for chronic respiratory disorders. Its product AridolR for the assessment of asthma is launched in a number of key markets. Its development pipeline of products includes: Bronchitol for cystic fibrosis, bronchiectasis, ASM8 for asthma, PXS25 for idiopathic pulmonary fibrosis and a new oxidase inhibitor for lung disease. Pharmaxis is listed on the Australian Securities Exchange (symbol PXS). The company is headquartered in Sydney at its TGAapproved manufacturing facilities. For more information about Pharmaxis, go to www.pharmaxis.com.au or contact Investor Relations on phone +61 2 9454 7200.
Forward-Looking Statements
Forward-looking statements in this media release include statements regarding our expectations, beliefs, hopes, goals, intentions, initiatives or strategies, including statements regarding the potential for Aridol and/or Bronchitol. All forward-looking statements included in this media release are based upon information available to us as of the date hereof, and we assume no obligation to update any such forward-looking statement as a result of new information, future events or otherwise. We cannot guarantee that any product candidate will receive regulatory approval or that we will seek any such approval.
| © Copyright 1996-2019 irasia.com Ltd. All rights reserved. |
|
DISCLAIMER: irasia.com Ltd makes no guarantee as to the accuracy or completeness of any
information provided on this website. Under no circumstances shall irasia.com Ltd be liable
for damages resulting from the use of the information provided on this website.
TRADEMARK & COPYRIGHT: All intellectual property rights subsisting in the contents of this website belong to irasia.com Ltd or have been lawfully licensed to irasia.com Ltd for use on this website. All rights under applicable laws are hereby reserved. Reproduction of this website in whole or in part without the express written permission of irasia.com Ltd is strictly prohibited. TERMS OF USE: Please read the Terms of Use governing the use of our website. |