
Living Cell Technologies Limited


Living Cell Technologies Limited
Company Announcement
17 May 2012: Sydney, Australia & Auckland, New Zealand - Living Cell Technologies Limited (ASX: LCT; OTCQX: LVCLY) has received approval from the New Zealand Minister of Health to trial a higher dose of its DIABECELL® product.
- Living Cell Technologies Limited (ASX: LCT; OTCQX: LVCLY) has received approval from the New Zealand Minister of Health to trial a higher dose of its DIABECELL® product.
A Phase 2 clinical trial of DIABECELL for treating unstable type 1 diabetes has been underway in New Zealand for two years. Establishing optimal dosage is a central aspect of the trial. This permission to proceed with the highest dose in two further patients will allow firm conclusions to be drawn about the optimum single dose. The New Zealand data combined with the results from a similar trial being conducted in Argentina, which uses two sequential doses, will ensure the optimum design of a pivotal trial. The company expects to start this in Q1 2013.
Professor Bob Elliott, LCT's Medical Director, said, "This approval, with additional ethical approval, allows us to build a more complete picture of the best procedure. Our preliminary report presented in Prague last year, which suggested more benefit from some doses than others, can be crystallised."
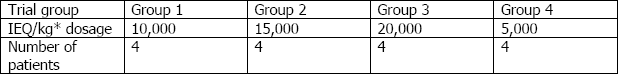
LCT's Phase II clinical trial groups for DIABECELL
For further information: www.lctglobal.com
| At the company: Dr Andrea Grant, Chief Executive Tel: +64 9 270 7941 Mobile: +64 21 078 5421 agrant@lctglobal.com Prof Bob Elliott Tel: +64 9 276 2690 |
Media enquiries: Sally Raudon Botica Butler Raudon Partners Tel: +64 9 303 3862 Mobile: +64 21 402 502 sallyr@botica.co.nz |
About Living Cell Technologies
LCT leads the world in cell transplant research and has implemented a business model that supports the discovery and advancement of products through preclinical and early clinical development. LCT aims to secure a major pharmaceutical partner to co-develop products through Phase II and pivotal studies, and ultimately market introduction. Value is returned to LCT principally through an ownership share of downstream product profits. LCT is incorporated in Australia. Research and development, operations and manufacturing facilities are based in New Zealand.
LCT Disclaimer
This document contains certain forward-looking statements, relating to LCT's business, which can be identified by the use of forward-looking terminology such as "promising," "plans," "anticipated," "will", "project", "believe", "forecast", "expected", "estimated", "targeting", "aiming", "set to," "potential," "seeking to," "goal," "could provide," "intends," "is being developed," "could be," "on track," or similar expressions, or by express or implied discussions regarding potential filings or marketing approvals, or potential future sales of product candidates. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. There can be no assurance that any existing or future regulatory filings will satisfy the FDA's and other health authorities' requirements regarding any one or more product candidates nor can there be any assurance that such product candidates will be approved by any health authorities for sale in any market or that they will reach any particular level of sales. In particular, management's expectations regarding the approval and commercialization of the product candidates could be affected by, among other things, unexpected clinical trial results, including additional analysis of existing clinical data, and new clinical data; unexpected regulatory actions or delays, or government regulation generally; our ability to obtain or maintain patent or other proprietary intellectual property protection; competition in general; government, industry, and general public pricing pressures; and additional factors that involve significant risks and uncertainties about our products, product candidates, financial results and business prospects. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described herein as anticipated, believed, estimated or expected. LCT is providing this information and does not assume any obligation to update any forward-looking statements contained in this document as a result of new information, future events or developments or otherwise.
| © Copyright 1996-2019 irasia.com Ltd. All rights reserved. |
|
DISCLAIMER: irasia.com Ltd makes no guarantee as to the accuracy or completeness of any
information provided on this website. Under no circumstances shall irasia.com Ltd be liable
for damages resulting from the use of the information provided on this website.
TRADEMARK & COPYRIGHT: All intellectual property rights subsisting in the contents of this website belong to irasia.com Ltd or have been lawfully licensed to irasia.com Ltd for use on this website. All rights under applicable laws are hereby reserved. Reproduction of this website in whole or in part without the express written permission of irasia.com Ltd is strictly prohibited. TERMS OF USE: Please read the Terms of Use governing the use of our website. |